PIPELINE
CUR101
CUR101 is a gene therapy in development designed to efficiently facilitate cell fusion between stem cells and other cells. The primary indication for this therapy will be Duchenne muscular dystrophy (DMD); over time, indications will be expanded to various muscle disorders and other diseases.
Duchenne muscular dystrophy (DMD)
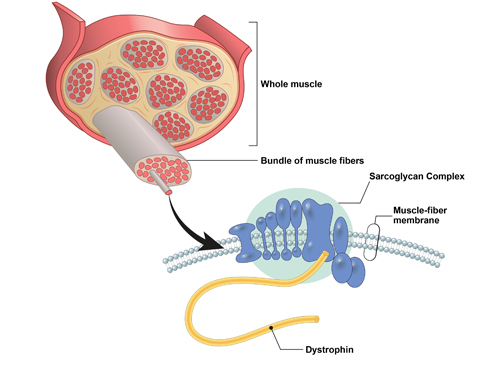
DMD is a genetic disorder characterized by progressive muscle degeneration and weakness due to the absence of the dystrophin protein caused by a genetic mutation. This condition was first identified in the 1830s, but later described in detail by the French neurologist Guillaume Benjamin Amand Duchenne in 1861. Importantly, however, little was known about the cause of DMD until the 1980s, and in 1987, researchers identified that a genetic abnormality of the dystrophin protein causes this condition. The disease primarily affects men because the mutated gene is located on the X chromosome. Women also occasionally develop relatively mild symptoms and are usually DMD carriers.
Symptoms
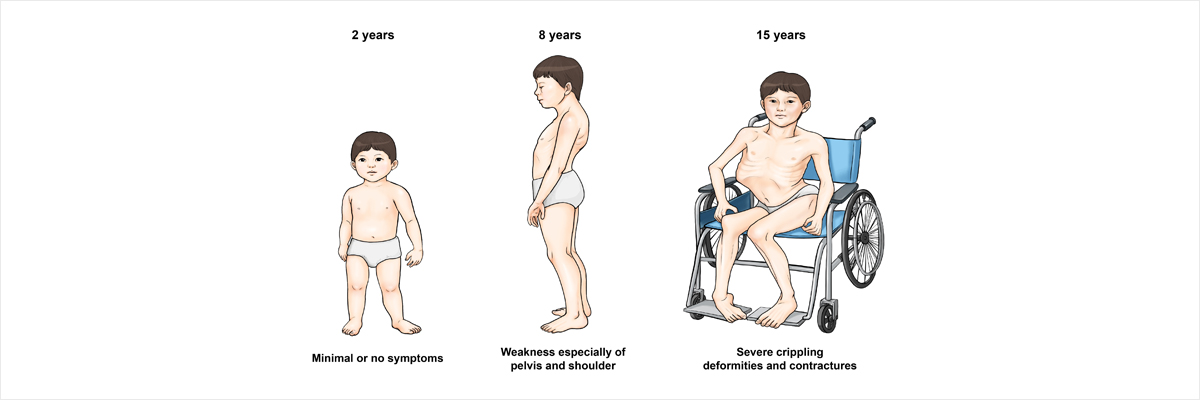
Muscle weakness is the principal symptom of DMD and symptom onset is usually between ages 3 and 5, gradually affecting the muscles in the hip, pelvis and shoulder regions. An affected child might have difficulty standing up, walking, and running. Most children with DMD may be slower to walk and often develop enlargement of calf muscles known as pseudohypertrophy. As this symptom progresses, affected children exhibit a waddling gait and present with difficulties in getting up from the sitting position and arms up in their primary years (elementary age). Typically, children with DMD require the use of a wheelchair for mobility at 10 years-old. From this age, the heart and respiratory muscles weaken as well. Since muscle weakness progresses very slowly, those affected initially complain of headache, nightmares, and insomnia before complaining of shortness of breath. Some children may manifest learning disabilities due to impairment of brain functions. In particular, impaired cardiac and respiratory functions most significantly affect these children and determines disease prognosis.
Diagnosis
In most cases, DMD can be preliminarily diagnosed based on inquiry, physical examination and family history. However, a confirmed diagnosis may require blood tests, electromyography, genetic tests, a muscle biopsy and other exams. In recent years, genetic confirmation has become most important and it is warranted to confirm whether a patient’s female siblings are carriers of a DMD gene mutation. A female who is a carrier of a DMD-causing mutation has a 50 percent chance of having a son affected by DMD and a 50 percent chance of having a daughter who is a carrier. The dystrophin gene is the largest known gene in humans, and various types of mutations have been reported in this gene. Since not all mutations are easily detected in general genetic testing, a thorough genetic exam and assessment of dystrophin protein expression via muscle biopsy may be needed to confirm a diagnosis of DMD.
Cause
DMD is caused by mutations in the dystrophin gene. Dystrophin is a protein complex that is vital in connecting the cytoskeleton of a muscle fiber to connective tissues outside of the cells. As a result, the lack of dystrophin leads to progressive muscle fiber damage during muscle contractions. Damaged muscle fibers result in inflammation and are replaced with fibrosis and fat as dystrophin abnormalities slow down muscle regeneration, eventually leading to progressive muscle degeneration. In patients with DMD, dystrophin is completely absent due to mutations in the dystrophin gene, whereas dystrophin is present, but in reduced amounts, in Becker muscular dystrophy (BMD), a less severe disease compared with DMD. Since the dystrophin gene is on the X chromosome, DMD occurs more frequently in men because males have only one X chromosome (XY for males) and are thus unable to compensate for the genetic defect. On the contrary, women are typically carriers of DMD because they have two X chromosomes, thus allowing them to compensate for a deficiency of the dystrophin gene on one X chromosome. Importantly, however, some female carriers can manifest symptoms of the disease.
Treatment
Collaborative care by a multidisciplinary team from different departments (e.g., neurology, rehabilitation medicine, pulmonology, cardiology, nutrition) is important to determine prognosis and prompt appropriate responses to patient’s symptoms. As the disease progresses, the use of assistive respiratory devices and cardiovascular medications is vital for management of difficulty breathing and cardiomyopathy. Since DMD can also be associated with learning disabilities, patients with learning problems need to be assisted. Corticosteroids are the main drug treatment for DMD. In multiple clinical trials, corticosteroids have been shown to delay disease progression by slowing down muscle weakness and maintaining respiratory function. However, since undesirable side effects are associated with the long-term use of corticosteroids in children, professional care is needed. In recent years, antisense oligomers (ASO) were developed and have been used in a limited number of patients with DMD. Considering their effectiveness and relatively high cost, ASOs are currently suboptimal.

 TOP
TOP